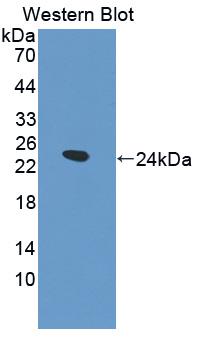
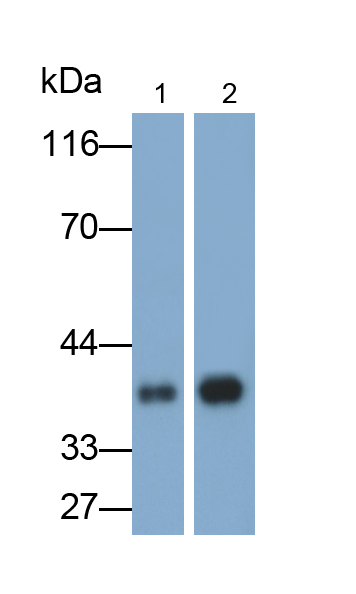
抑瘤素M(OSM)多克隆抗体
Polyclonal Antibody to Oncostatin M (OSM)
OS-M
- 编号PAA110Hu06
- 物种Homo sapiens (Human,人)相同的名称,不同的物种。
- 来源多抗制备
- 宿主兔
- 效价-
- Ig类型IgG
- 纯化方式抗原特异性亲和纯化
- 标记物无标记物
- 免疫原-
- 缓冲液成份磷酸盐缓冲液(pH7.4, 含有 0.01% SKL, 1mM DTT, 5% Trehalose和Proclin300.)
- 性状液体
- 浓度500µg/mL
- 且适物种Rattus norvegicus (Rat,大鼠)
- 应用WB,IHC
如果抗体需用于流式细胞术,请参见流式抗体。 - 下载英文说明书 中文说明书
- 规格20µl100µl200µl1ml10ml
- 价格¥ 565¥ 1319¥ 1884¥ 4710¥ 18840
- 欲了解实际交易价格和更多情况,请与当地经销商联系!
特异性
该抗体是针对OSM的兔多克隆抗体。在免疫组织化学染色和免疫印迹实验中能识别OSM。
用法
免疫印迹:0.2-2µg/mL;1:250-2500
免疫组织化学:5-20µg/mL;1:25-100
免疫细胞化学:5-20µg/mL;1:25-100
最佳稀释倍数最终由用户决定。
储存
经常使用则4°C保存。-20°C保存不超过两年。避免反复冻融。
稳定性
热稳定性以损失率显示。损失率是由加速降解试验决定,具体方法如下:在37°C孵育48小时,没有显著的降解或者沉淀产生。保质期内,在适当的条件下存储,损失率低于5%。
赠品
增值服务
相关产品
| 编号 | 适用物种:Homo sapiens (Human,人) | 应用(仅供研究使用,不用于临床诊断!) |
| EPA110Hu61 | 抑瘤素M(OSM)真核蛋白 | Positive Control; Immunogen; SDS-PAGE; WB. |
| RPA110Hu01 | 抑瘤素M(OSM)重组蛋白 | Positive Control; Immunogen; SDS-PAGE; WB. |
| APA110Hu61 | 抑瘤素M(OSM)活性蛋白 | Cell culture; Activity Assays. |
| APA110Hu01 | 抑瘤素M(OSM)活性蛋白 | Cell culture; Activity Assays. |
| PAA110Hu06 | 抑瘤素M(OSM)多克隆抗体 | WB,IHC |
| PAA110Hu01 | 抑瘤素M(OSM)多克隆抗体 | WB; IHC; ICC; IP. |
| MAA110Hu22 | 抑瘤素M(OSM)单克隆抗体 | WB; IHC; ICC; IP. |
| SEA110Hu | 抑瘤素M(OSM)检测试剂盒(酶联免疫吸附试验法) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| SCA110Hu | 抑瘤素M(OSM)检测试剂盒(化学发光免疫分析法) | Chemiluminescent immunoassay for Antigen Detection. |
| LMA110Hu | 抑瘤素M(OSM)等多因子检测试剂盒(流式荧光发光法) | FLIA Kit for Antigen Detection. |
| KSA110Hu01 | 抑瘤素M(OSM)检测试剂盒DIY材料(酶联免疫吸附试验法) | Main materials for "Do It (ELISA Kit) Yourself". |
参考文献
| 杂志 | 参考文献 |
| Journal of Obstetrics and Gynaecology Research | Oncostatin M as a target biological molecule of preeclampsia[PubMed: 20149034] |
| J Neuroinflammation | Oncostatin M promotes excitotoxicity by inhibiting glutamate uptake in astrocytes: implications in HIV-associated neurotoxicity via Smad2/4 in Human Renal Proximal Tubular Epithelial Cells[Pubmed:27287400] |
| 22 | Modulating the phenotype of host macrophages to enhance osteogenesis in MSC-laden hydrogels: Design of a glucomannan coating material.[pubmed:28582717] |
| Advanced Functional Materials | Fungal Component Coating Enhances Titanium Implant‐Bone Integration[Doi: 10.1002/adfm.201804483] |