MAPK/ERK信号通路:从基础生物学到人类疾病的核心枢纽
MAPK/ERK信号通路:从基础生物学到人类疾病的核心枢纽
1. MAPK通路简介
丝裂原活化蛋白激酶(MAPK)级联反应是调节多种细胞过程的关键信号通路,包括增殖、分化、细胞凋亡和应激反应。MAPK通路通过信号级联发挥作用,将细胞外信号传递到细胞内靶标,使细胞能够对各种特定的细胞外刺激做出反应。MAPK通路包括三种主要激酶,即MAPK激酶激酶(MAP3K)、MAPK激酶(MAPKK)和MAPK,它们激活和磷酸化下游蛋白质。目前的研究发现有四种主要且不同的MAPK级联反应:细胞外信号调节激酶1和2(ERK1/2)、c-Jun N端激酶(1、2和3)、p38 MAPK(α、β、γ和δ)和ERK5。本文重点介绍MAPK/ERK信号通路。
1.1 MAPK/ERK通路功能
MAPK/ERK通路是一条至关重要的细胞信号传导途径,其核心作用是将细胞外的生长因子信号(如促增殖、分化指令)逐级放大并传递至细胞核内,通过激活特定的转录因子来调控基因表达,从而主导细胞的增殖、分化、存活和代谢等多种关键生物学过程。ERK通路中上游蛋白质和激酶的过度激活已被证明会诱发各种疾病,包括癌症、炎症、发育障碍和神经系统疾病。此外,MAPK/ERK通路在器官再生过程中具有核心作用。MAPK/ERK信号传导响应损伤刺激而迅速激活,并协调促再生机制,包括细胞存活、迁移、增殖、生长以及相关基因的转录和翻译。
1.2 MAPK/ERK通路激活方式
MAPK/ERK通路主要通过配体刺激质膜上的受体酪氨酸激酶(RTK)来激活,也可以被G蛋白偶联受体(GPCR)激活。然后,RTK信号通过生长因子受体结合蛋白2(Grb2)和SOS传递,激活小GTP酶Ras,招募Ras和Ser/Thr激酶Raf到质膜,形成复合物,通过诱导Raf上丝氨酸残基的磷酸化/去磷酸化来激活Raf。活性Raf依次磷酸化并激活MEK1/2。MEK1/2分别对ERK1/2蛋白进行磷酸化从而激活ERK1/2。ERK1/2通过不同亚细胞区室中的磷酸化激活或灭活多种蛋白质,也可以通过磷酸化多个转录因子靶点,快速穿梭到细胞核中调节细胞转录活性。此外,ERK1/2可以作为负反馈调控机制,磷酸化ERK通路的上游激酶,如SOS和MEK。
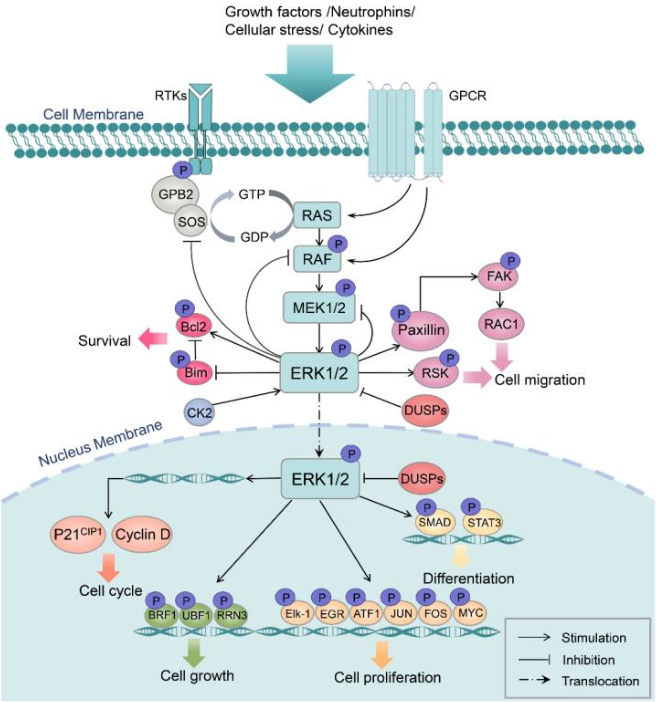
图1 MAPK/ERK通路调控机制和功能的简化示意图
(图片源于《Int J Mol Sci》[1])
2. MAPK/ERK通路与肿瘤的相关研究
MAPK/ERK通路功能障碍是多种癌症发展的主要诱因之一。多项研究发现MAPK/ERK信号通路的激活可促进结直肠癌(CRC)[2]、乳腺癌[3]、卵巢癌[4,5]、肝癌[6]、小细胞肺癌[7]、甲状腺癌[8]、胃癌[9]等癌症的发生、增殖、迁移和侵袭。直接或间接抑制MAPK/ERK信号传导可抑制肿瘤的增殖和迁移,并减弱恶性表型[10-12]。MAPK/ERK信号与肿瘤治疗耐药性相关[13,14]。与这些结果相反,MAPK/ERK通路是胶质母细胞瘤(GB)细胞对抗肿瘤免疫敏感性的关键调节因子[15]。GB细胞中实验诱导的ERK磷酸化提高了免疫检查点阻断(ICB)治疗的存活率,重新激发并产生持久的抗肿瘤免疫。此外,多种化合物通过MAPK/ERK通路在肿瘤中发挥促细胞凋亡作用[16-18]。这些研究表明MAPK/ERK通路激活是肿瘤进展的一把“双刃剑”,突出了其在肿瘤治疗中的潜在价值。
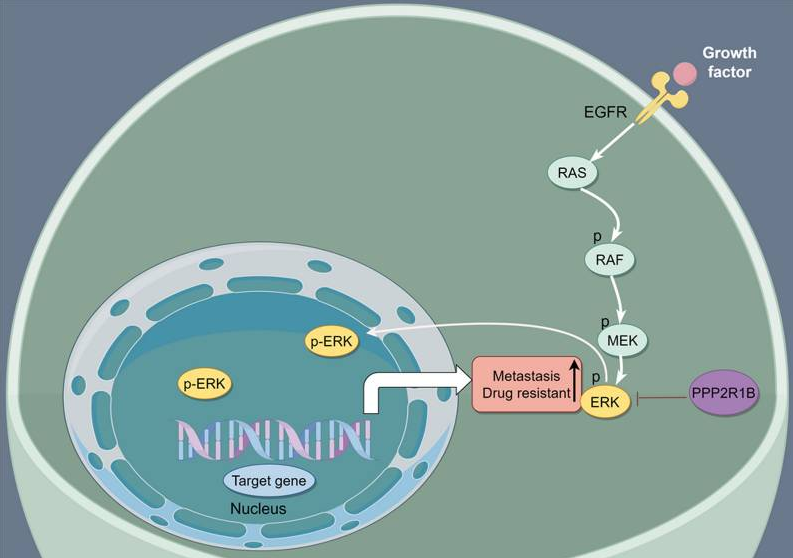
图2 PPP2R1B通过MAPK/ERK信号通路促进CRC细胞对奥沙利铂的敏感性
(图片源于《Cancer Cell Int》[14])
3. MAPK/ERK通路与自身免疫疾病的相关研究
MAPK/ERK在自身免疫性疾病中的作用受到广泛研究。MAPK/ERK通路介导类风湿性关节炎成纤维细胞样滑膜细胞(RA-FLS)的增殖和迁移,并可能有助于RA的进展[19]。抑制MAPK/ERK信号通路,可减少FLS增殖并减轻RA滑膜炎程度[20]。精胺通过以MAPK/ERK依赖性方式抑制CD4 T细胞活化和T效应细胞分化来缓解多发性硬化症疾病模型进展[21]。绿原酸可抑制葡聚糖硫酸钠(DSS)诱导的结肠炎症,改善结肠黏膜中MAPK/ERK通路相关蛋白的表达[22]。ERK抑制剂逆转了绿原酸对结肠组织的保护作用。黄芩苷正丁酯通过结合ERK蛋白和抑制ROS/ERK/P-ERK/NLRP3信号通路抑制细胞焦亡,预防小鼠结肠炎[23]。在系统性硬化症中,IL11依赖性ERK信号传导介导真皮成纤维细胞激活,促进纤维化表型[24]。这些结果表明靶向MAPK/ERK通路可能是一种有前景的自身免疫疾病治疗方法。
4. MAPK/ERK通路与心血管疾病
MAPK/ERK信号通路的异常激活广泛参与心血管疾病的发生发展。在压力超负荷诱导下,ERK1/2磷酸化并激活ETS2,与NFAT形成复合物,驱动心脏肥大[25]。抑制MAPK/ERK信号传导可抑制心肌细胞的进一步肥大[26]。MAPK/ERK通路表达下调可预防AngII诱导的小鼠心脏肥大[27]。研究显示ERK1/2信号传导是早期弹性蛋白酶激活的重要调节剂,其药理学抑制可能阻止主动脉瓣疾病(AVD)进展[28]。穿心莲内酯通过MAPK-ERK信号通路抑制细胞增殖来改善主动脉瓣增生[29]。磷酸化ERK表达增加对心肌缺血/再灌注损伤具有保护作用,减轻心肌梗死面积,减少心肌细胞细胞凋亡[30,31]。这些结果为精准靶向MAPK-ERK来预防和治疗心血管疾病提供帮助。
5. MAPK/ERK通路与神经退行性疾病
MAPK/ERK通路是神经退行性疾病发展过程中与神经炎症相关的重要通路。研究显示人参皂苷Rg2对阿尔茨海默病(AD)的神经保护作用可能与MAPK-ERK通路有关[32]。抑制MAPK/ERK通路可逆转Aβ1-42肽对神经干细胞/祖细胞(NSPC)迁移的抑制作用,改善NSPC对AD的治疗效果[33]。帕金森(PD)小鼠模型中抑制MAPK信号传导可挽救神经元细胞死亡和运动功能障碍[34]。抑制MAPK/ERK途径可减少LRRK2突变(帕金森病发病原因之一)细胞系中的异常自噬和细胞凋亡[35]。MAPK/ERK信号调节缺氧诱导的自噬过程,从而改善SOD1突变(肌萎缩侧索硬化症发病原因之一)运动神经元活力[36]。因此,探索MAPK信号通路的特异性调控机制可能为开发神经退行性疾病的新治疗药物提供线索。
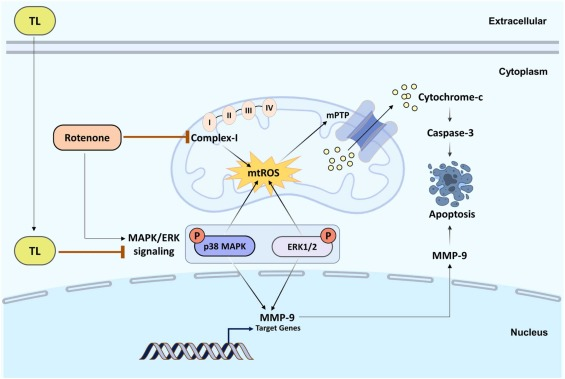
图3 盐酸托哌酮诱导PD神经保护的机制
(图片源于《Biomed Pharmacother》[34])
6. MAPK/ERK通路与再生
越来越多的研究强调了MAPK/ERK通路在组织和器官再生过程中的重要作用。MAPK/ERK信号通路在电离辐射后的造血重建中发挥重要作用[37]。低振幅电场通过激活MAPK/ERK通路调节内皮血管生成,促进血管组织修复[38]。适当激活MAPK/ERK信号传导有助于斑马鱼心脏再生[39]。MAPK/ERK通路的激活有效促进牙周骨再生,并取得良好的恢复效果[40]。三七皂苷R1可以促进MAPK/ERK信号通路的激活,下调TNF-α的表达,最终上调成骨基因的表达,增强骨再生[41]。数据表明,MAPK/ERK通路调控肝祖细胞(HPC)的细胞增殖和集落形成,是肝脏再生的关键通路[42]。这些证据突出了靶向MAPK/ERK通路诱导组织和器官再生能力的可能性和潜力。
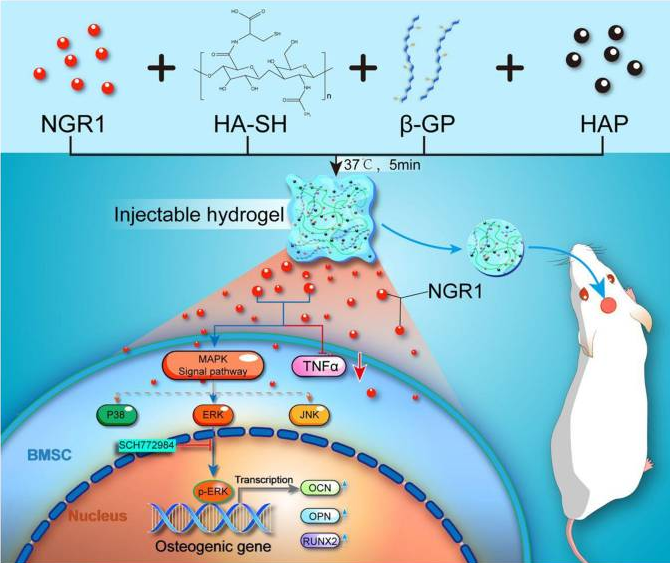
图4 三七皂苷R1促进骨再生
(图片源于《Front Bioeng Biotechnol》[41])
云克隆助力科学研究,为广大科研人员提供相关检测试剂产品,相关靶标核心货号如下:
靶标 | 核心货号 | 靶标 | 核心货号 | 靶标 | 核心货号 |
MAPK1 | A930 | MAP3K1 | B145 | FOS | B291 |
MAPK3 | B357 | MAP3K5 | B358 | GRB2 | C514 |
MAPK6 | D566 | MAP3K6 | D558 | JUN | B292 |
MAPK7 | B431 | MAP3K7 | D567 | MEF2A | C647 |
MAPK8 | B156 | MAP3K12 | D572 | MYC | B290 |
MAPK9 | D576 | MAP4K1 | D551 | PAK1 | H469 |
MAPK10 | B869 | MAP4K5 | B135 | PAK2 | H468 |
MAPK11 | B435 | MAPKAPK2 | B460 | RAC1 | M427 |
MAPK12 | D577 | MAPKAPK3 | B632 | RAF1 | C232 |
MAPK13 | D578 | DUSP1 | C902 | RASA1 | B616 |
MAPK14 | B206 | DUSP5 | F975 | RPS6KA1 | M085 |
MAP2K1 | D559 | DUSP6 | F976 | RPS6KA5 | M090 |
MAP2K2 | D562 | DUSP3 | F973 | SHC1 | E671 |
MAP2K3 | D563 | DUSP9 | F979 | TRADD | M390 |
MAP2K4 | D564 | ATF4 | B385 | TRAF2 | G752 |
MAP2K6 | B721 | CDC42 | E614 | TRAF6 | G751 |
MAP2K7 | D560 | DAXX | C259 |
更多科研试剂,欢迎访问云克隆官方网站:http://www.cloud-clone.cn/
参考文献
[1]Wen X, Jiao L, Tan H. MAPK/ERK Pathway as a Central Regulator in Vertebrate Organ Regeneration. Int J Mol Sci. 2022;23(3):1464.
[2]Bai X, Wei H, Liu W, et al. Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut. 2022;71(12):2439-2450.
[3]Wu J, Li J, Xu H, Qiu N, Huang X, Li H. Periostin drives extracellular matrix degradation, stemness, and chemoresistance by activating the MAPK/ERK signaling pathway in triple-negative breast cancer cells. Lipids Health Dis. 2023;22(1):153.
[4]Ma H, Qi G, Han F, Gai P, Peng J, Kong B. HMGB3 promotes the malignant phenotypes and stemness of epithelial ovarian cancer through the MAPK/ERK signaling pathway. Cell Commun Signal. 2023;21(1):144.
[5]Chen K, Liu MX, Mak CS, et al. Methylation-associated silencing of miR-193a-3p promotes ovarian cancer aggressiveness by targeting GRB7 and MAPK/ERK pathways. Theranostics. 2018;8(2):423-436.
[6]Huang K, Liu Z, Xie Z, et al. HIGD2A silencing impairs hepatocellular carcinoma growth via inhibiting mitochondrial function and the MAPK/ERK pathway. J Transl Med. 2023;21(1):253.
[7]Wang Z, Kan G, Sheng C, Yao C, Mao Y, Chen S. ARHGEF19 regulates MAPK/ERK signaling and promotes the progression of small cell lung cancer. Biochem Biophys Res Commun. 2020;533(4):792-799.
[8]Zhang HM, Li ZY, Dai ZT, et al. Interaction of MRPL9 and GGCT Promotes Cell Proliferation and Migration by Activating the MAPK/ERK Pathway in Papillary Thyroid Cancer. Int J Mol Sci. 2022;23(19):11989.
[9]Ma F, Yao J, Niu X, Zhang J, Shi D, Da M. MARK4 promotes the malignant phenotype of gastric cancer through the MAPK/ERK signaling pathway. Pathol Res Pract. 2024;261:155471.
[10]Hou J, Chen Q, Huang Y, Wu Z, Ma D. Caudatin blocks the proliferation, stemness and glycolysis of non-small cell lung cancer cells through the Raf/MEK/ERK pathway. Pharm Biol. 2022;60(1):764-773.
[11]Zhang H, Liu J, Dang Q, et al. Ribosomal protein RPL5 regulates colon cancer cell proliferation and migration through MAPK/ERK signaling pathway. BMC Mol Cell Biol. 2022;23(1):48.
[12]Li J, Hu S, Zhang Z, Qian L, Xue Q, Qu X. LASP2 is downregulated in human liver cancer and contributes to hepatoblastoma cell malignant phenotypes through MAPK/ERK pathway. Biomed Pharmacother. 2020;127:110154.
[13]Peng WX, Huang JG, Yang L, Gong AH, Mo YY. Linc-RoR promotes MAPK/ERK signaling and confers estrogen-independent growth of breast cancer. Mol Cancer. 2017;16(1):161.
[14]Liu W, Tang J, Gao W, Sun J, Liu G, Zhou J. PPP2R1B abolishes colorectal cancer liver metastasis and sensitizes Oxaliplatin by inhibiting MAPK/ERK signaling pathway. Cancer Cell Int. 2024;24(1):90.
[15]Kim KS, Zhang J, Arrieta VA, et al. MAPK/ERK signaling in gliomas modulates interferon responses, T cell recruitment, microglia phenotype, and immune checkpoint blockade efficacy. Preprint. bioRxiv. 2024;2024.09.11.612571.
[16]Jeon SJ, Choi EY, Han EJ, et al. Piperlongumine induces apoptosis via the MAPK pathway and ERK‑mediated autophagy in human melanoma cells. Int J Mol Med. 2023;52(6):115.
[17]An J, Li L, Zhang X. Curcusone C induces apoptosis in endometrial cancer cells via mitochondria-dependent apoptotic and ERK pathway. Biotechnol Lett. 2021;43(1):329-338.
[18]Yano S, Wu S, Sakao K, Hou DX. Involvement of ERK1/2-mediated ELK1/CHOP/DR5 pathway in 6-(methylsulfinyl)hexyl isothiocyanate-induced apoptosis of colorectal cancer cells. Biosci Biotechnol Biochem. 2019;83(5):960-969.
[19]Liu F, Feng XX, Zhu SL, et al. Sonic Hedgehog Signaling Pathway Mediates Proliferation and Migration of Fibroblast-Like Synoviocytes in Rheumatoid Arthritis via MAPK/ERK Signaling Pathway. Front Immunol. 2018;9:2847.
[20]Chen J, Luo X, Liu M, et al. Silencing long non-coding RNA NEAT1 attenuates rheumatoid arthritis via the MAPK/ERK signalling pathway by downregulating microRNA-129 and microRNA-204. RNA Biol. 2021;18(5):657-668.
[21]Zheng R, Kong M, Wang S, He B, Xie X. Spermine alleviates experimental autoimmune encephalomyelitis via regulating T cell activation and differentiation. Int Immunopharmacol. 2022;107:108702.
[22]Gao W, Wang C, Yu L, et al. Chlorogenic Acid Attenuates Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice through MAPK/ERK/JNK Pathway. Biomed Res Int. 2019;2019:6769789.
[23]Guo M, Wu H, Zhang J, et al. Baicalin n-butyl ester alleviates inflammatory bowel disease and inhibits pyroptosis through the ROS/ERK/P-ERK/NLRP3 pathway in vivo and in vitro. Biomed Pharmacother. 2025;186:118012.
[24]Adami E, Viswanathan S, Widjaja AA, et al. IL11 is elevated in systemic sclerosis and IL11-dependent ERK signalling underlies TGFβ-mediated activation of dermal fibroblasts. Rheumatology (Oxford). 2021;60(12):5820-5826.
[25]Luo Y, Jiang N, May HI, et al. Cooperative Binding of ETS2 and NFAT Links Erk1/2 and Calcineurin Signaling in the Pathogenesis of Cardiac Hypertrophy. Circulation. 2021;144(1):34-51.
[26]Ye J, Yan S, Liu R, et al. CMTM3 deficiency induces cardiac hypertrophy by regulating MAPK/ERK signaling. Biochem Biophys Res Commun. 2023;667:162-169.
[27]Hu B, Song JT, Ji XF, Liu ZQ, Cong ML, Liu DX. Sodium Ferulate Protects against Angiotensin II-Induced Cardiac Hypertrophy in Mice by Regulating the MAPK/ERK and JNK Pathways. Biomed Res Int. 2017;2017:3754942.
[28]Munjal C, Jegga AG, Opoka AM, et al. Inhibition of MAPK-Erk pathway in vivo attenuates aortic valve disease processes in Emilin1-deficient mouse model. Physiol Rep. 2017;5(5):e13152.
[29]Huang Y, Liu M, Liu C, Dong N, Chen L. The Natural Product Andrographolide Ameliorates Calcific Aortic Valve Disease by Regulating the Proliferation of Valve Interstitial Cells via the MAPK-ERK Pathway. Front Pharmacol. 2022;13:871748.
[30]Chen Y, Ba L, Huang W, et al. Role of carvacrol in cardioprotection against myocardial ischemia/reperfusion injury in rats through activation of MAPK/ERK and Akt/eNOS signaling pathways. Eur J Pharmacol. 2017;796:90-100.
[31]Fu C, Wang M, Lu Y, et al. Polygonum orientale L. Alleviates Myocardial Ischemia-Induced Injury via Activation of MAPK/ERK Signaling Pathway. Molecules. 2023;28(9):3687.
[32]Ye X, Shao S, Wang Y, Su W. Ginsenoside Rg2 alleviates neurovascular damage in 3xTg-AD mice with Alzheimer's disease through the MAPK-ERK pathway. J Chem Neuroanat. 2023;133:102346.
[33]Wang Z, Chen Y, Li X, Sultana P, Yin M, Wang Z. Amyloid-β1-42 dynamically regulates the migration of neural stem/progenitor cells via MAPK-ERK pathway. Chem Biol Interact. 2019;298:96-103.
[34]Zaman B, Mostafa I, Hassan T, et al. Tolperisone hydrochloride improves motor functions in Parkinson's disease via MMP-9 inhibition and by downregulating p38 MAPK and ERK1/2 signaling cascade. Biomed Pharmacother. 2024;174:116438.
[35]Bravo-San Pedro JM, Niso-Santano M, Gómez-Sánchez R, et al. The LRRK2 G2019S mutant exacerbates basal autophagy through activation of the MEK/ERK pathway. Cell Mol Life Sci. 2013;70(1):121-136.
[36]D'Amico AG, Maugeri G, Saccone S, et al. PACAP Modulates the Autophagy Process in an In Vitro Model of Amyotrophic Lateral Sclerosis. Int J Mol Sci. 2020;21(8):2943.
[37]Yang L, Lu Y, Zhang Z, et al. Oxymatrine boosts hematopoietic regeneration by modulating MAPK/ERK phosphorylation after irradiation-induced hematopoietic injury. Exp Cell Res. 2023;427(2):113603.
[38]Sheikh AQ, Taghian T, Hemingway B, Cho H, Kogan AB, Narmoneva DA. Regulation of endothelial MAPK/ERK signalling and capillary morphogenesis by low-amplitude electric field. J R Soc Interface. 2013;10(78):20120548.
[39]Liu P, Zhong TP. MAPK/ERK signalling is required for zebrafish cardiac regeneration. Biotechnol Lett. 2017;39(7):1069-1077.
[40]Chen H, Liu N, Hu S, et al. Yeast β-glucan-based nanoparticles loading methotrexate promotes osteogenesis of hDPSCs and periodontal bone regeneration under the inflammatory microenvironment. Carbohydr Polym. 2024;342:122401.
[41]Liu Y, Zhang Y, Zheng Z, et al. Incorporation of NGR1 promotes bone regeneration of injectable HA/nHAp hydrogels by anti-inflammation regulation via a MAPK/ERK signaling pathway. Front Bioeng Biotechnol. 2022;10:992961.
[42]Jin C, Samuelson L, Cui CB, Sun Y, Gerber DA. MAPK/ERK and Wnt/β-Catenin pathways are synergistically involved in proliferation of Sca-1 positive hepatic progenitor cells. Biochem Biophys Res Commun. 2011;409(4):803-807.